Ebola, Chimeras, and Unexpected Speculation
If you were studying Ebola before 2014, chances are that you wouldn’t have heard of brincidofovir, an antiviral drug created by a North Carolina company called Chimerix. Within the span of a few weeks in October and November 2014, however, brincidofovir became one of the most promising Ebola countermeasures in clinical development. The story of the drug’s path to the front lines of the Ebola crisis underscores the contingent, speculative, “chimeric” nature of contemporary global health.
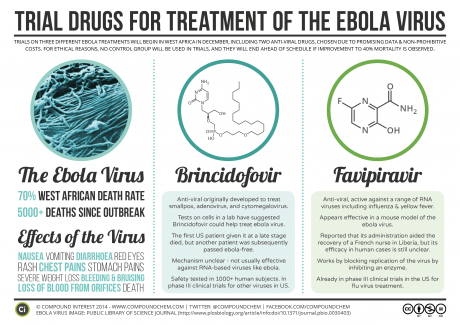
Brincidofovir was never meant to be an Ebola drug. It was designed, in part, as a smallpox therapy. Commercial drug makers have had vaccines and drugs for smallpox and Ebola in their pipelines for some time, but the market has tended to be too small to attract much private investment. Although humanitarian groups have publicly encouraged (and financially pushed) pharmaceutical companies to develop treatments for “neglected diseases,” until very recently, they gave Ebola minimal attention in these efforts (McGoey et al. 2001).
In fact, U.S. biopreparedness programs were paying more attention to Ebola, which joined smallpox on a list of “select agents.” In the wake of a series of international bioterror attacks in the 1990s, the U.S. Department of Defense (DOD) began investing in pharmaceutical countermeasures to potential bioterror threats, including anthrax, smallpox, and Ebola. After the 2001 anthrax scare, the National Institute for Allergy and Infectious Diseases (NIAID)—a domestic agency of the Department of Health and Human Services (HHS)—saw an increase in its budget for biothreat research. Both DOD and NIAID used this money to entice private pharmaceutical companies to develop drugs and vaccines, but by the end of the George W. Bush administration, billions in (largely uncoordinated) military and civilian investments failed to move most potential countermeasures out of the laboratory and into late-stage clinical trials. This became painfully clear in 2014 when supplies of ZMapp, a drug that showed potential against Ebola in early animal studies, quickly ran out. Even though HHS had founded the Biomedical Advanced Research and Development Authority (BARDA) in 2006 to move drugs like ZMapp from labs to clinical development more efficiently, the agency’s funding had consistently fallen short of the totals necessary for full-scale tests (Greeley and Chen 2014).
Amid this series of bureaucratic and appropriations missteps, the government did have a few successes. Brincidofovir was one of them. It was just the kind of drug that the government’s medical countermeasure programs were initiated to support, a therapy that would be effective in patients already infected with a virus: the smallpox virus. Chimerix received millions in funding, first from the NIAID and later from BARDA. The company fought vigorously for BARDA’s attention, even filing a complaint with the U.S. Government Accountability Office (GAO) in 2011 when BARDA inserted an option into its contract with a competitor firm to purchase millions of extra doses of its prospective smallpox therapy. The GAO upheld Chimerix’s complaint. The company used the language of biopreparedness and market fairness to justify the complaint, saying that the GAO’s decision to nullify BARDA’s option to buy extra doses of the competing product “allows BARDA the opportunity to competitively procure a second smallpox antiviral, consistent with the U.S. government’s long-stated strategy of having two smallpox antiviral drugs for protecting the public against the intentional or unintentional release of the smallpox virus” (Chimerix 2011).
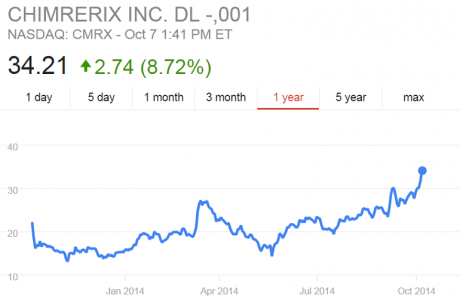
Brincidofovir is a “prodrug,” a weakened form of the antiviral cidofovir. It becomes fully active only when human bodies begin to metabolize it. This makes it potentially more suitable in patients already infected with smallpox and other DNA viruses. Until recently, no one had considered brincidofovir’s efficacy in patients infected with RNA viruses like Ebola. In early 2014, however, Chimerix was asked to provide brincidofovir to the U.S. Centers for Disease Control (CDC) and the National Institutes of Health (NIH) to determine its efficacy against Ebola. Somewhat surprisingly, it showed high potency against the RNA virus in culture (Kroll 2014b). In October 2014, with the ZMapp failure making headlines and other therapies such as Tekmira’s TKM-Ebola causing worry about harsh side effects, Chimerix received U.S. Food and Drug Administration (FDA) approval to test brincidofovir in U.S. patients with Ebola (Racaniello 2014). One of the first recipients was Thomas Eric Duncan, the Liberian man who remains the only person to die of Ebola on U.S. soil (Kroll 2014a). At the time of these American tests, one financial analyst noted that Chimerix “shares [were] likely to be (awkwardly) tracking the fate of the Ebola outbreak” (Tirrell 2014). Indeed, by November, the company’s stock rose from a midyear low of $14 per share to $35 per share, and the company raised more than $121 million in a stock offering (Chimerix 2014a).
Chimerix’s approach to Ebola trials for brincidofovir was initially domestic and defensive. As the company’s chief medical officer explained when the trials were announced, “Our objective is really for us to determine what the safety and antiviral activity is of brincidofovir when used to treat Ebola virus, and really in the setting of the U.S., where we have patients that are basically being relocated from the West African theater, or in patients…who presented with Ebola virus disease in the U.S.” (Loftus 2014; emphasis added). The use of the military term “theater” here is telling. BARDA’s interest in smallpox (for which brincidofovir was to be a countermeasure) stemmed in part from a wartime mindset: in the early 2000s, smallpox attack scenarios were at the heart of a civilian-cum-military biopreparedness complex (Lakoff 2008). While a “natural” disease outbreak such as the 2014 Ebola event was one of the many scenarios for which planners had prepared, at no point did BARDA couch smallpox (or anthrax or Ebola) as a matter of humanitarian concern. Rather, it was framed as a threat to U.S. lives and property. Testing it in the context of a medical infrastructure that had been preparing for more than a decade to address a novel biothreat from a neglected pathogen seemed most appropriate. Chimerix claimed it needed that infrastructure to carry out its trial, and its public communications expressed uncertainty about “whether brincidofovir is effective in the West African theater” (Loftus 2014).
Just one month later, however, on November 13, Chimerix announced that brincidofovir would be one of two drugs used in a clinical Ebola trial in West Africa, operated with the support of Oxford University and the Oxford-based International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) (Chimerix 2014b). ISARIC, an initiative to facilitate open-access protocols and data sharing in clinical research on acute respiratory diseases including SARS, bird flu, and swine flu, is partnering with Médecins Sans Frontieres (MSF; Doctors Without Borders), a humanitarian organization that has been confronting Ebola outbreaks for decades, to help design and plan the trial. ISARIC’s pivot from its focus on respiratory diseases like SARS to viral hemorrhagic fevers like Ebola was propelled by a WHO initiative and a 3.2-million-pound grant from the Wellcome Trust (Wellcome Trust 2014). Less than a week later, the Bill and Melinda Gates Foundation committed $5.7 million to a similar multidrug trial in Africa, also including brincidofovir (Bracken 2014). Brincidofovir’s prodrug design made it highly portable and suitable for oral administration. In addition, the drug’s history of human clinical trial success (albeit in trials against DNA viruses) made it a good bet (Kroll 2014b).
Chimerix, a pharmaceutical firm once seeded by BARDA to build countermeasures against domestic biothreats, was now joining the world’s most prominent humanitarian medical institutions: MSF and the Gates Foundation. Thus, brincidofovir has drifted from one pharmaceutical infrastructure to another: it has been partially disembedded from the biopreparedness complex in which it was incubated, and moved to the humanitarian complex where it provides hope. The company’s leaders now find themselves in novel ethical territory. Chimerix’s CEO told attendees at a biotech conference in December 2014 that “innovation [is] not just in the products, but in the trial design.” Brincidofovir will not be tested in randomized control trials with placebo, but in adaptive trials that provide treatment to all infected patients (Oleniacz 2014). Meanwhile, MSF is pushing Ebola drugmakers, including Chimerix, to scale up production of their drugs in advance of trial completion (Moran 2014).

Unexpected Chimera.
This unexpected institutional recombination contains a few lessons for critical scholars of global health. A clue lies in the proto-mythological company name, “Chimerix.” The root word “chimera” can connote something spectral or elusive. For many years, the spread of Ebola beyond isolated “hot zones” in Africa’s interior was thought to be unlikely without a significant genetic mutation (Preston 1994). In the United States, stories of Ebola epidemics in such zones enabled speculation about whether a mutant strain might wreak havoc on political and economic stability, but most actual epidemics seemed manageable using response kits devised by MSF. As Peter Redfield notes in this issue of Limn, MSF’s kits helped contain outbreaks, but they could do little to stem the progression of Ebola in infected patients. BARDA was established to develop countermeasures in the event Ebola or another pathogen started to spread at a larger scale, whether through a terror attack or through a “natural” mutation. In addition, a variety of American funders—from USAID to DOD to Google—have begun to support “virus-hunting” projects to identify new pathogens before they emerge. In 2014, the Ebola-related deaths of thousands of West Africans (far outside the original central African “hot zone”) revealed that the “global” reach of this predictive biosecurity infrastructure was itself somewhat spectral and elusive. Ebola mutates frequently, but there is no clear evidence that a mutation caused the current crisis. It seems just as likely that transformations in the West African landscape (including deforestation and road building) have combined with increased human mobility and a chronic deficiency in public health infrastructure to make human-to-human transmission possible (Nguyen 2014; Street 2014).
The word “chimera” also refers to a multiheaded monster. Responses to global health crises tend to be governed by what Andrew Lakoff (2010) has called “two regimes,” that of humanitarianism and biosecurity. These operations are sometimes enabled (and sometimes hampered) by a third regime: pharmaceutical capitalism. The efforts of the DOD, NIAID, and BARDA to seed the work of companies such as Chimerix (efforts supplemented by the Wellcome Trust and Gates Foundation in the latest Ebola crisis) are one example. Larger corporations like GlaxoSmithKline and Johnson & Johnson, who have had Ebola vaccines in their pipelines for years, have also benefitted from a resurgence of philanthropic, investor, and government support (The Economist 2014).
What the story of brincidofovir reveals is that the institutional assemblages of global health operate as much in contingency and chance as in planning and preparedness. The story of the drug’s journey from prospective domestic smallpox countermeasure to the front lines of the African Ebola crisis is less one of concerted corporate “rollout” than of recursive scenes of threat and response, sudden promise and enthusiastic investment. Chimeras—monstrous, hybrid, or simply fantastical—tend to be figures of liminality. Their importance is heightened in moments when someone or something sits betwixt and between social categories and states of being (Turner 1964). Drugs like brincidofovir appear promising, but are still short of assured. Likewise, Ebola’s shift from potential domestic biothreat to global humanitarian concern is far from complete. If the crisis abates, or if media and lawmakers become crisis-weary, we may see Ebola’s profile shift again, from humanitarian concern back to biothreat (and, as the financial pages remind us, Chimerix’s stock price may suffer as a result). This uncertainty illustrates the chimeric nature of global health, a network of sites and practices in which crisis is how we come to know life, and how life becomes capital.